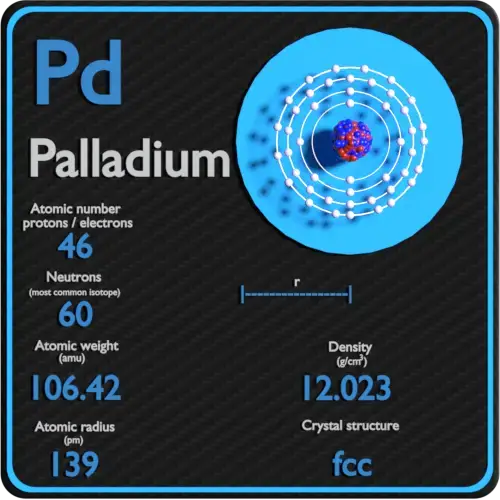
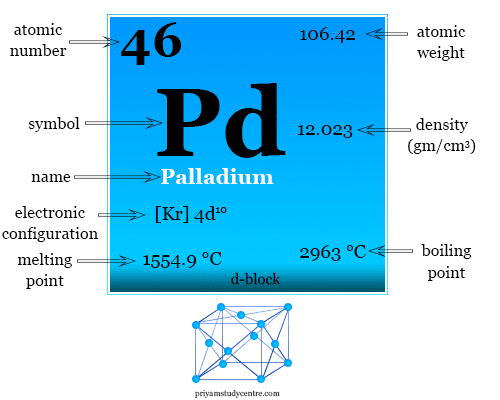
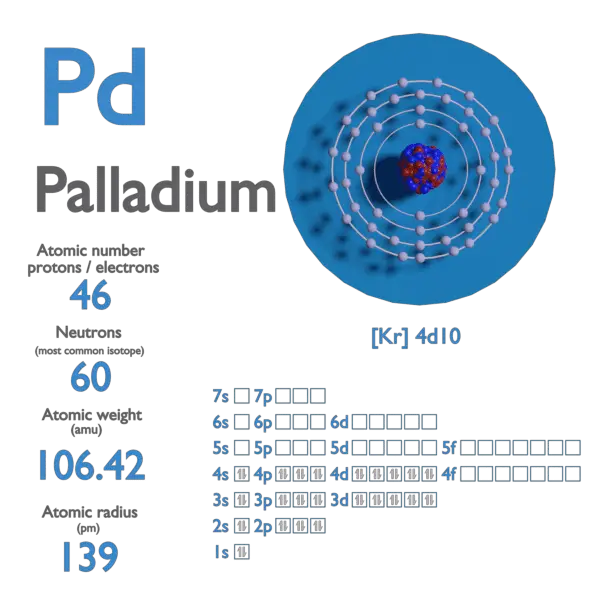
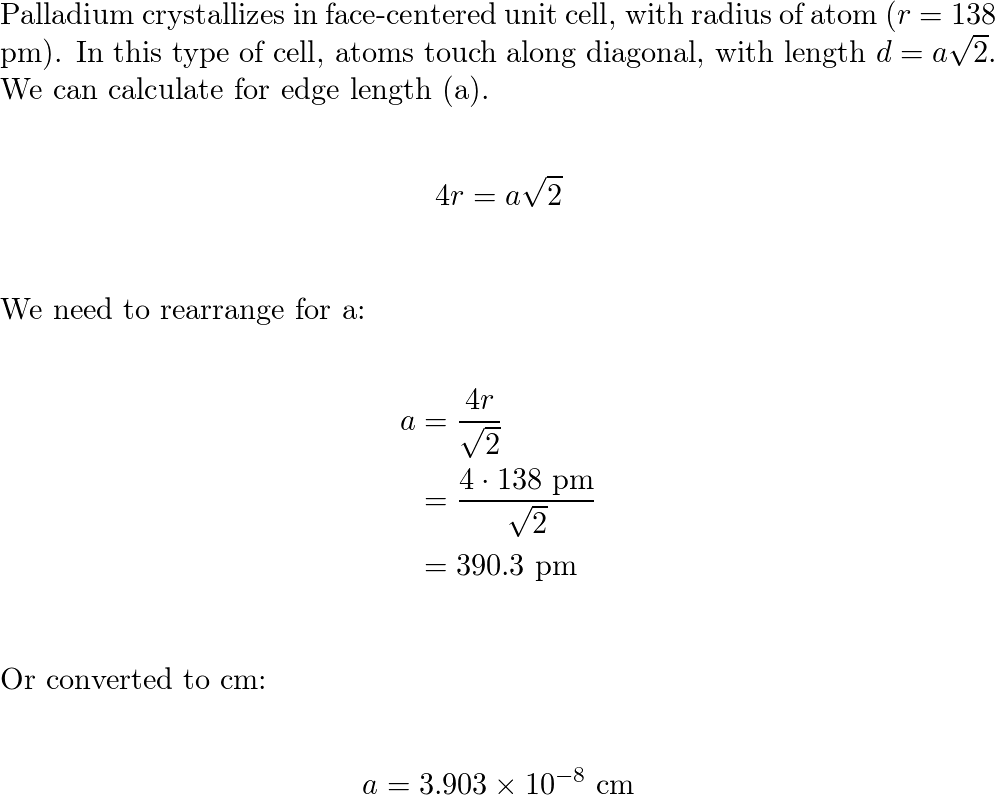Document - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium. | Course Hero
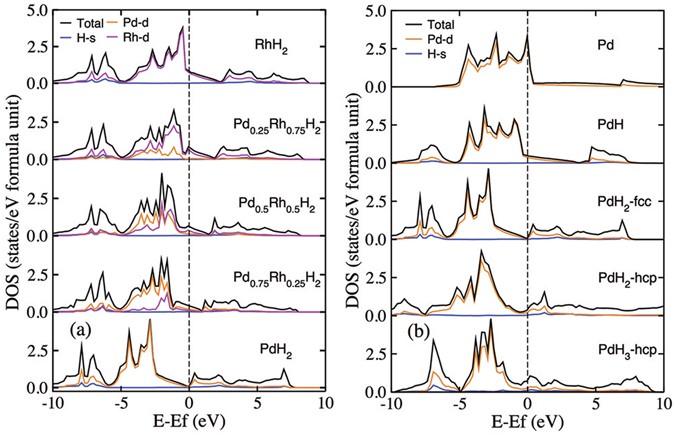
Formation and electronic properties of palladium hydrides and palladium-rhodium dihydride alloys under pressure | Scientific Reports

0.39" Element Cube Palladium 10mm Density Cubes for Periodic Table Collection High Purity Element Collections (0.39", Palladium)| | - AliExpress

the density of palladium is 12.0g/Cm^3. what volume in liters would be occupied by 532 g of - Brainly.com